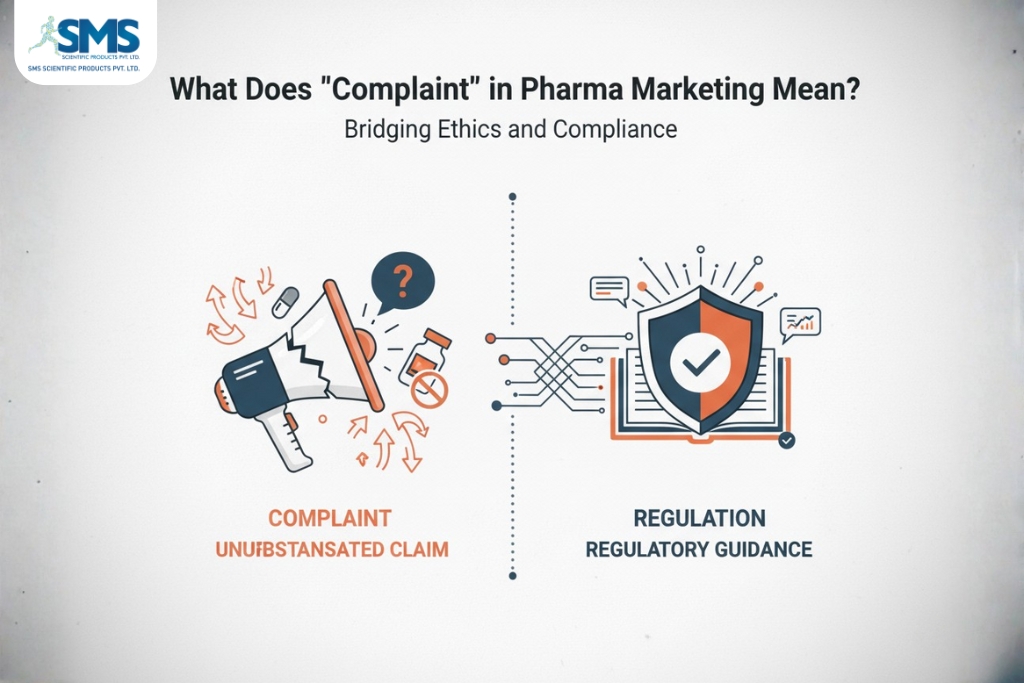
In pharma marketing, a complaint is any report or concern raised about a product, its promotion, or how it is marketed. This includes quality issues (damaged packs, wrong label), safety reports (adverse drug reactions), and alleged breaches of marketing codes (misleading claims or unethical promotion). Front Life Sciences+1
Companies must treat complaints seriously because they can affect patient safety, regulatory compliance, and brand trust. Typical complaint handling follows four steps: receive and log, investigate technically, take corrective and preventive actions (CAPA), and report findings to relevant authorities when needed. Good practices and SOPs for complaint management are standard in Indian pharma. Pharmaguideline+1
For safety-related complaints (adverse reactions), healthcare professionals and patients in India report to the Pharmacovigilance Programme of India (PvPI) or the CDSCO using designated ADR forms and hotlines—this is mandatory for serious events. CDSCO+1
Promotional or marketing complaints (e.g., ethical breaches) are handled under codes such as the Uniform Code for Pharmaceutical Marketing Practices (UCPMP) and industry bodies like OPPI, which provide complaint portals and ethics committees. indiaoppi.com+1
Conclusion: Clear, documented complaint handling protects patients and a brand’s reputation. SMS Scientific integrates compliant, evidence-based materials and quality controls in its pharma-branding products—helping clients reduce promotional-risk, support doctor trust, and strengthen long-term brand recall.